Chemical Equation for the Synthesis of Aspirin
In the presence of moisture aspirin may decompose hydrolysis into salicylic acid and acetic acid. Acetylsalicylic acid binds.
D 2 Synthesis Of Aspirin Sl Youtube
Limiting Reagent Synthesis Of Aspirin.
. The reaction using molecular formulas is C7H6O3 C4H6O3 -- C9H8O4 C2H4O2. Mcmaster University Chem2o06 Lab Manual. Once aspirin is synthesized it needs to be purified and.
Calculate the percent yield if you collect 225 g of product at the end of the reaction. 8-Synthesis of Aspirin. Salicylic acid acetic anhydride Acetylsalicylic acid Acetic acid C7H6O3 C4H6O3 C9H8O4 C2H4O2 0.
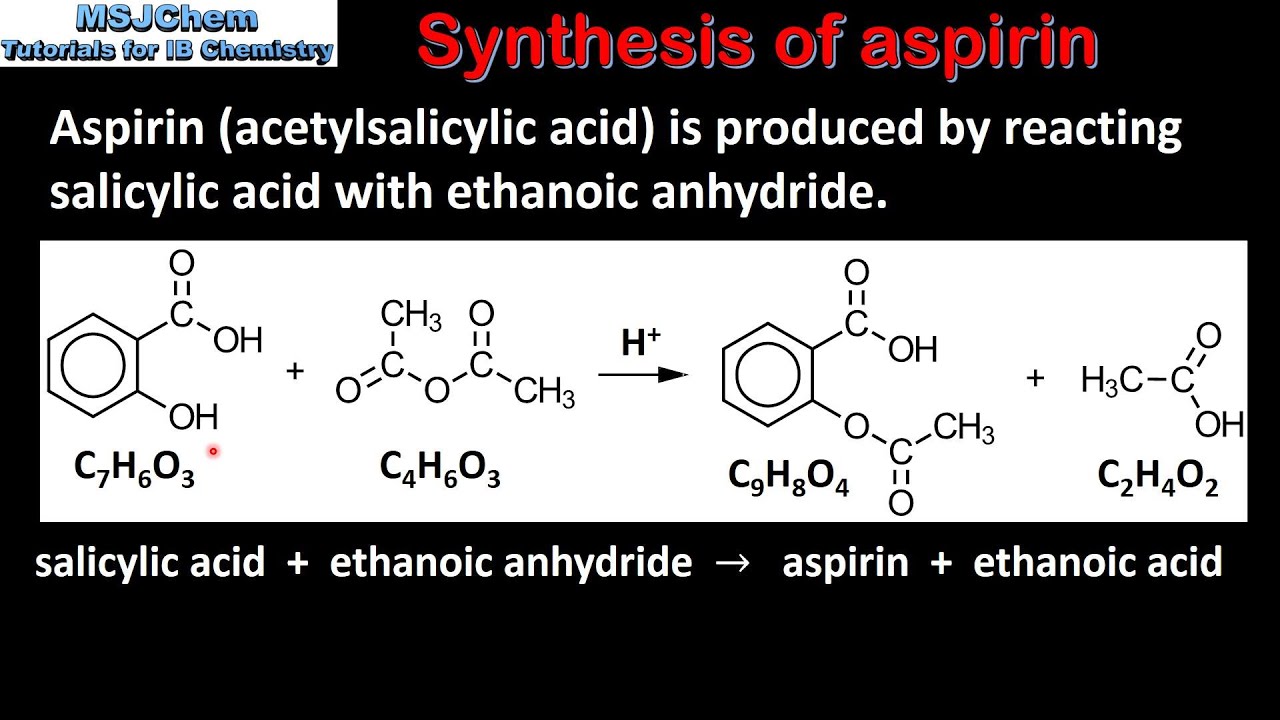
In the synthesis of aspirin sulfuric acid is also used as a catalyst to speed up the reaction Wikipedia Sulfuric acid 2013. The chemical equation for the synthesis of aspirin is C7H6O3 C4H6O3 C9H8O4 C2H4O2 which is a reaction of salicylic acid with acetic anhydride in the presence of phosphoric acid. The mechanism for this synthesis provides examples of three major classes of chemical reactions.
H 2 SO 4 use a dropper H 2 SO 4 is highly corrosive and. This carbonyl group can undergo typical carbonyl addition-elimination reactions. The chemical name for Aspirin is Acetylsalicylic Acid.
Hydrolysis condensation and proton transfer. A balanced equation for the synthesis of Aspirin from Salicylic Acid and Acetic Anhydride is the following. Chemistry questions and answers.
Balance the equation for synthesis salicylic acid acetic anhydride chemistry of aspirin formulas formula mechanism organic how to synthesize without using. Aspirin is prepared by chemical synthesis from salicylic acid through acetylation with acetic anhydride. Anti-inflammatory inhibition of the synthesis of prostaglandins.
Transfer the impure aspirin from the Büchner funnel to a 150-mL beaker. The structures below will all be tested on the TLC plates after you make your aspirin. 6 C 6 H 5 OH Fe3 FeOC 6 H 5 63 6 H phenol violet complex If your produ ct is pure no violet color will be observed since the aspirin cannot form the violet complex not a phenol.
Chemical Formula of Aspirin Acetylsalicylic acid C 9 H 8 O 4. The Chemistry of Aspirin acetylsalicylic acid Chemical formula C9H8O4 or CH3COOC6H4COOH or HC9H7O4. The molecular weight of aspirin is 18016gmol.
SYNTHESIS OF ASPIRIN acetylsalicylic acid Place 20 g 0015 mole of salicylic acid in a 125-mL Erlenmeyer flask. The mixture can be heated gently until the solid. Why Is Acetic Anhydride Used In Aspirin Synthesis Socratic.
Limiting Reagent â Synthesis Of Aspirin Faculty Web Pages Suny. The byproduct is acetic acid. Methyl Salicylate is a phenol with a acetyl group in the ortho position.
The two main functional groups in aspirin are. Calculate the percent yield for the above reaction if the amount of aspirin obtained was 2301 g. 3623 mol 0.
C 7 H 6 O 3 CH 3 CO 2 O C 9 H 8 0 4 CH 3 COOH. Synthesis And Characterization Of Aspirin. Synthesis of Aspirin Synthesis Purification Characterization.
The chemical equation corresponding to the synthesis reaction is. Synthesis of Aspirin. 3623 mol 0.
Aspirin is an orally administered non-steroidal antiinflammatory agent. Aspirin Synthesis Mechanism Organic Chemistry You. Molecular formula of salicylic acid C 7 H 6 O 3 Molecular formula of acetylsalicylic acid aspirin C 9 H 8 O 4 Molecular weight of salicylic acid 138 gmole.
The Synthesis Of A Medicinal Agent Aspirin By Walter Scharf And Charles Malerich Natural Sciences Chemistry Baruch College New. If 35 0 G Of Ch Oh Mm. CH 3 COOC 6 H 4 COOH.
What is the chemical equation for the synthesis of aspirin. Methyl Salicylate is a naturally occurring chemical that is obtained from winter green oil that can be used to synthesize Salicylic Acid. IronIII reacts with phenols to form a violet-colored complex.
Organic Synthesis Of Aspirin From Benzene The Science Snail. Using the equation for the synthesis of aspirin in the introduction calculate the theoretical yield of aspirin 1. Limiting reactants are important in chemical reactions because a reaction cannot proceed without all of the reactants.
Adding cold water stops the reaction and the products are filtered yielding aspirin. The chemical equation for the synthesis of aspirin. This reaction is the reverse of the synthesis reaction.
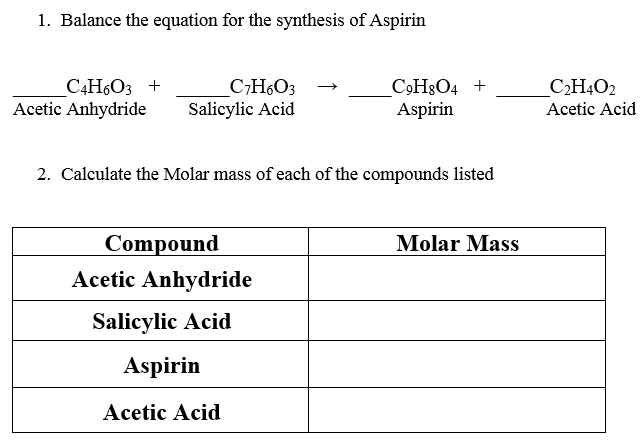
Write out the balanced equation for the reaction between salicylic acid and acetic anhydride. The synthesized aspirin can be determined by reaction of the product with Fe3. It is the two-step synthesis of aspirin starting from oil of wintergreen.
Up to 24 cash back 1 mol aspirin 18017 g aspirin 00305 mols salicylic acid 550 g aspirin 1 mol salicylic acid 1 mol aspirin 392 g Yield 100 713 550 g. C7H6O3 C4H6O3 C9H8O4 C2H4O2 2. That is to say a reaction can only occur until one reactant is used up Kirk 2013.
Salicylic acid Acetic anhydride Aspirin Acetic acid Please use this balanced equation and the weight of salicylic acid used to calculate the theoretical yield. Add about 25 mL of warm 50-70 C distilled water and stir to dissolve the impure aspirin. Add 10 mL of 95 ethanol.
Aspirin product as well as a commercial aspirin tablet will be compared to a standard 015 ferric-salicylate solution. The relevant molecular weights are 180 grams per mole for aspirin and 138 grams per mole for salicylic acid. Add 5 mL 005 mole of acetic anhydride followed by 5 drops of conc.
From 200 g of starting material. The maximum allowable amount of. Extended Molecular Formula of Aspirin.
Patented by Bayer in 1893. Gastric irritation bleeding. An experiment is described that is suitable for the early portion of the laboratory in a general chemistry course and integrates organic examples.
The Chemistry Of Aspirin The International Aspirin Foundation

Solved Balance The Equation For The Synthesis Of Aspirin Chegg Com
No comments for "Chemical Equation for the Synthesis of Aspirin"
Post a Comment